Tryp advantages
Tryp’s pipeline differs from other psychedelic drug development companies in three important ways.
Unique Indications
The first wave of psychedelic drug development has been focused on mental health disorders such as depression, anxiety, addiction and PTSD.
As the leader in next-wave development, Tryp is evaluating psilocybin-based drug products for chronic pain and other indications with multiple Phase 2a trials initiating in 2021.
Competitive Landscape: Psychedelic Drug Development Companies
Tryp is one of just a handful of psychedelic drug development companies that will be conducting Phase 2 clinical trials in 2021 as shown in the following landscape:

While mental health indications such as depression, anxiety and addiction are crowded by multiple companies, Tryp Therapeutics is breaking new ground in chronic pain and other indications.
A proprietary formulation and delivery method.
Our psilocybin-based drug product, TRP-8803, features a proprietary formulation and novel method of administration to improve the patient experience. We have filed a provisional patent to protect the unique features of TRP-8803.

An exclusive supply chain.
We’ve secured an exclusive supply of our psilocybin-based active ingredients and drug products, with sufficient quantities of material to support clinical trials and ultimate commercialization—a rare capability among psychedelic drug development companies.
Our Active Pharmaceutical Ingredient (API) and final drug products are manufactured in the U.S., circumventing clinical trial import restrictions.
Our vertical integration will allow us to supply the international research community with synthetic psilocybin.
Tryp employs current good manufacturing processes (cGMP) to meet the requirements of FDA-approved drugs.
We’re on the cusp of initiating our Phase 2 clinical trials.
As part of our Psilocybin For Neuropsychiatric Disorders (PFN™) program, we’re currently testing two drugs for efficacy and patient experience: TRP-8802 and TRP-8803.
Our novel method of administration for TRP-8803 is expected to improve the patient experience. Our drug products are given in tandem with psychotherapy before and after drug treatment.
Clinical trial timeline
TRP-8802
As Tryp Therapeutics is solely focused on psilocybin, and because more than 50 FDA-registered studies have firmly established the compound’s safety profile, we expect to bypass preclinical and Phase 1 studies and enter directly into Phase 2a clinical trials.
This non-proprietary 25 mg oral capsule of synthetic psilocybin will be used to determine initial efficacy of the drug product for our indications.

Clinical trial timeline
TRP-8803
This proprietary, psilocybin-based drug is manufactured exclusively for Tryp by Curia and Alcami and features a novel route of administration.
Bridging studies are being completed with the University of Michigan and other institutions to prepare TRP-8803 for use in Phase 2b clinical trials and beyond.

Psilocybin’s unique benefits
We seek to develop new, effective treatment options for patients without the adverse side effects of many alternatives.
Psilocybin’s potential to transform the treatment of several unique indicators has been researched by many well-respected academic institutions.

Chronic Pain Pipeline
The need is clear. So is the solution.
A closer look at patient needs.
Fibromyalgia
- Existing treatments have limited efficacy and significant side effects. Less than 10% of patients adhere to treatment after one year.
- Nearly 30% of fibromyalgia patients take opioids despite the lack of evidence of their efficacy and the risk of addiction and/or overdose.
- We estimate that there are approximately 4 million diagnosed cases of fibromyalgia in the United States.
Phantom Limb Pain

- There are nearly 2 million patients in the US living with the loss of a limb with more than 60% experiencing PLP at some point.
- PLP patients feel pain in limbs that no longer exist. As these pain signals are neurologically initiated, they are a prime target for treatment with psilocybin.
- Existing treatment methods such as general pain medication, mirror therapy and acupuncture have limited efficacy for many patients.
- We estimate that there are approximately 1 million diagnosed cases of PLP in the United States.
CRPS

- CRPS results from a misfiring of pain signals within the brain after an injury, surgery, stroke or heart attack.
- The brain becomes trained to signal pain at the time of injury and healing, but the pain signal persists even after the injury is fully healed and there is no local trauma.
- We estimate that there are nearly 100,000 diagnosed cases of CRPS in the United States.
We’re collaborating with top experts and research centers.
We’ve partnered with the Chronic Pain & Fatigue Research Center at the University of Michigan to advance our fibromyalgia studies—and we will be announcing partnerships for our clinical trials for phantom limb pain and complex regional pain syndrome soon.

Tryp’s clinical trial timeline for chronic pain.
Based on existing preclinical and clinical data for the active ingredients in TRP-8802, we anticipate FDA support to proceed directly into Phase 2 trials.
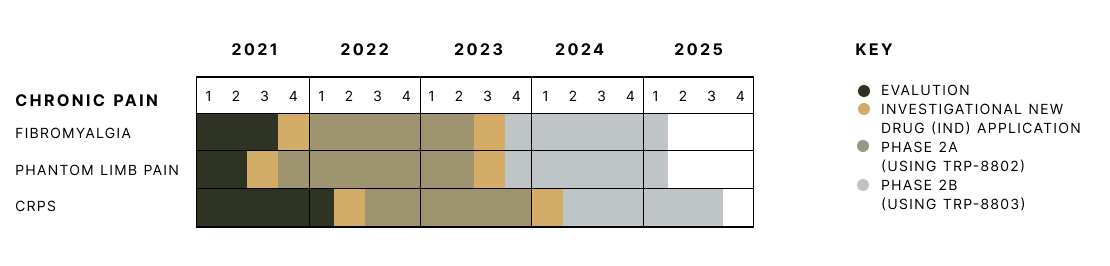
Eating Disorders Pipeline
For millions of patients, relief can’t come soon enough.
A closer look at patient needs.
Binge Eating Disorder (BED)

- BED is characterized by recurring episodes of eating large quantities of food and feeling unable to stop.
- Nearly 30% of people seeking weight loss treatments show signs of BED.
- Up to 3.5% of females and 2.0% of males will develop BED at some point in their lives.
Hypothalamic Obesity
- Hypothalamic obesity, a rare disease, is caused by damage to the hypothalamus, which controls hunger.
- This damage is often caused by the surgical removal of a brain tumor.
- This condition can dominate patients’ day-to-day lives, and with no FDA-approved therapies available for the condition, patients have few options.
- This condition can dominate patients’ day-to-day lives, and with no FDA-approved therapies available for the condition, patients have few options.
We’re collaborating with top experts and research centers.
Tryp is collaborating with Jennifer Miller, M.D. from the University of Florida’s Division of Pediatric Endocrinology to evaluate the efficacy of our drug products. Dr. Miller is a world-renowned expert in overeating disorders such as binge eating and hypothalamic obesity

Tryp’s clinical trial timeline for eating disorders.
Based on the well-established safety profile of psilocybin, we expect to advance directly into Phase 2a clinical trials.
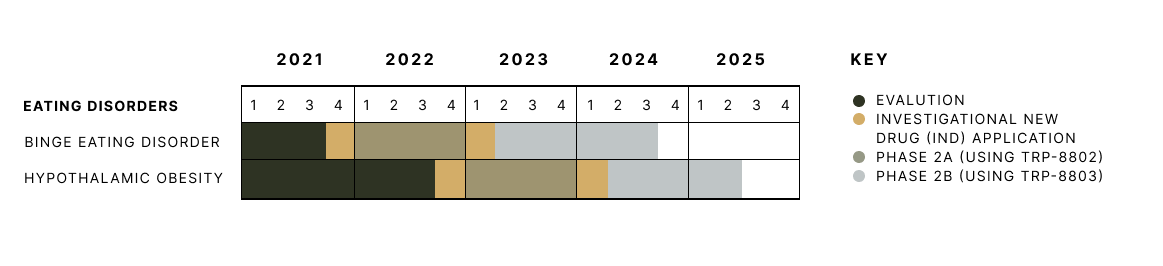
Manufacturing
Top partnerships & practices enable our success.
Tryp manufactures its own synthetic psilocybin—a unique asset among psychedelic drug development companies.
You can expect excellence at every stage.
Our Partners
Curia (formerly AMRI) manufactures our Active Pharmaceutical Ingredient (API) through a proprietary process.
Alcami manufactures and develops analytical methods for our final drug products.
Working at scale
We’ve already completed engineering batches for our API, and expect to have more than 2 kg of cGMP material in 2021.
We are already scaled for full commercial quantities.
Current good manufacturing practice (cGMP)
We employ cGMP in our manufacturing, the FDA standard for ensuring pharmaceutical quality.
Adherence to cGMP regulations assures the identity, strength, quality and purity of our drug products.
Manufacturing flow
Our manufacturing process at a glance.
We have established a unique manufacturing process to support our clinical trials and to supply drug products to patients once commercialized.

Operational excellence is at the core of all we do at Tryp Therapeutics in our pursuit to provide effective treatments to millions of patients around the world.