Our goal is to alleviate patient suffering.
Over a decade of research into psychedelics is expected to deliver safer and more effective treatments for depression, addiction, anxiety and other mental health conditions.
There is another wave coming.
We are moving psychedelics beyond mental health. Our mission at Tryp is to turn research into patient-friendly treatments in areas with deep unmet needs—starting with chronic pain and eating disorders.
Each one of these conditions is a humanitarian need.
A well-established safety profile.
Tryp Therapeutics is focused solely on psilocybin. Its potential to treat chronic pain and other indications is established by industry studies and an understanding of how psilocybin affects the brain.
With more than 50 FDA-registered studies already completed, psilocybin has a firmly established safety profile with no risk of addiction.

We anticipate bypassing preclinical and Phase 1 clinical trials for our treatments, cutting years off of development times.
A better experience for patients.
Alongside the development of our own synthetic version of psilocybin, our objective is to maximize patient benefit while minimizing patient discomfort through our innovative formulation and method of delivery.
 Current state
Current state
-
+
Daily medications
-
+
Hard-to-live-with side effects
-
+
Treating the symptoms
 Our vision for the future
Our vision for the future
-
+
Treatment given once or twice a year
-
+
Patient-friendly drug administered with psychotherapy
-
+
Treating the disease
Understanding more about chronic pain
Persistent and debilitating pain, with few effective treatments.
Tryp Therapeutics is developing psilocybin-based drug products to address the origins of pain for fibromyalgia, phantom limb pain (PLP), and complex regional pain syndrome (CRPS) patients.
Fibromyalgia
- Existing treatments have limited efficacy and significant side effects. Less than 10% of patients adhere to treatment after one year.
- Nearly 30% of fibromyalgia patients take opioids despite the lack of evidence of their efficacy and the risk of addiction and/or overdose.
- We estimate that there are approximately 4 million diagnosed cases of fibromyalgia in the United States.
Phantom Limb Pain

- There are nearly 2 million patients in the US living with the loss of a limb with more than 60% experiencing PLP at some point.
- PLP patients feel pain in limbs that no longer exist. As these pain signals are neurologically initiated, they are a prime target for treatment with psilocybin.
- Existing treatment methods such as general pain medication, mirror therapy and acupuncture have limited efficacy for many patients.
- We estimate that there are approximately 1 million diagnosed cases of PLP in the United States.
CRPS

- CRPS results from a misfiring of pain signals within the brain after an injury, surgery, stroke or heart attack.
- The brain becomes trained to signal pain at the time of injury and healing, but the pain signal persists even after the injury is fully healed and there is no local trauma.
- We estimate that there are nearly 100,000 diagnosed cases of CRPS in the United States.
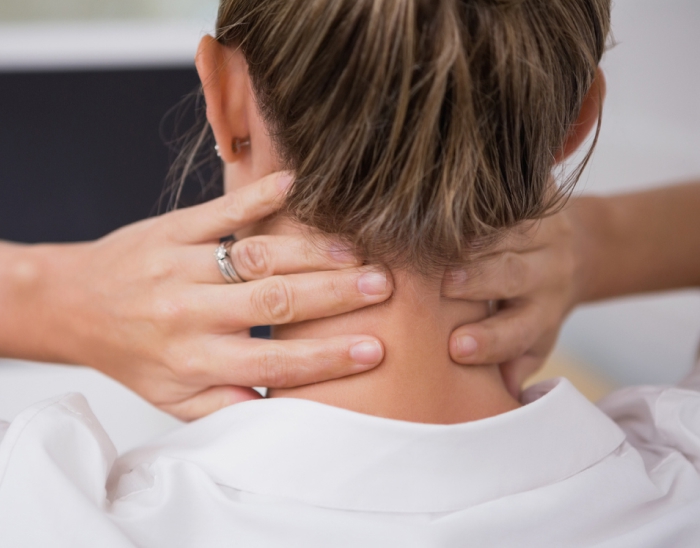
Tryp’s clinical trial timeline for chronic pain.
Chronic pain: a deeper look
In this video—and the interview transcript below—Kevin Boehnke, Ph.D., the Principal Investigator of our Phase 2a clinical trial for fibromyalgia, explains the potential of our work.
Kevin Boehnke, Ph.D., explains the potential of psilocybin.
If you are interested in participating in our upcoming Phase 2a clinical trial for fibromyalgia, then please provide your contact information so we can let you know once we start enrollment for the study.
Understanding more about eating disorders
An overwhelming need to eat that affects daily living.
Conditions including binge eating and hypothalamic obesity have limited treatment options. Both are expected to respond favorably to the neuroplasticity effects of our psilocybin treatment.
Binge Eating Disorder

- BED is characterized by recurring episodes of eating large quantities of food and feeling unable to stop.
- Nearly 30% of people seeking weight loss treatments show signs of BED.
- Up to 3.5% of females and 2.0% of males will develop BED at some point in their lives.
Hypothalamic Obesity
- HO refers to obesity that is caused by damage to the hypothalamus, which produces hormones that control hunger.
- The damage to the hypothalamus resulting in HO is often caused by the surgical removal of a brain tumor.
- There are no FDA-approved therapies available for hypothalamic obesity.
Tryp’s clinical trial timeline for eating disorders.
Tryp Therapeutics is developing psilocybin-based drug products to address the source of hunger signals for binge eating disorder and hypothalamic obesity.
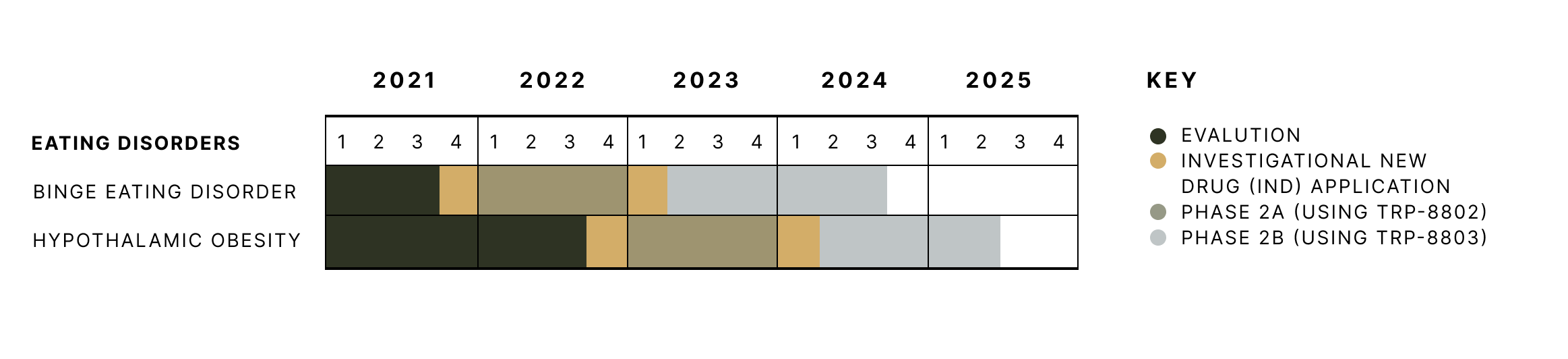
Eating disorders: a deeper look
In this video—and the interview transcript below—University of Florida Professor Jennifer Miller M.D. from the Division of Pediatric Endocrinology and the Principal Investigator of our Phase 2a clinical trial for eating disorders explains the potential of our work.
Professor Jennifer Miller M.D, explains the potential of psilocybin.
If you are interested in participating in our upcoming Phase 2a clinical trial for overeating disorders, then please provide your contact information so we can let you know once we start enrollment for the study.
You have questions. We have answers.
Download the Patient FAQ
